- Phosphorus [P] – Element Details, History, Atomic Structure, Facts, Properties, Electronic Configuration, Atomic Spectrum, Uses.
- Phosphorus
- History of Phosphorus
- How to Locate Phosphorus on Periodic Table
- Phosphorus Facts
- Phosphorus Atomic Structure and Orbital Properties
- Element Properties
- Atomic Structure of Phosphorus
- Crystal Structure of Phosphorus
- Ground State Electronic Configuration of Phosphorus - neutral Phosphorus atom
- Regulatory and Health - Health and Safety Parameters and Guidelines
- Phosphorus Chemical Properties : Phosphorus Ionization Energies and electron affinity
- Phosphorus Physical & Elastic Properties
- Phosphorus Electrical Properties
- Phosphorus Magnetic Properties
- Phosphorus Thermal Properties
- Use of Phosphorus
Phosphorus [P] – Element Details, History, Atomic Structure, Facts, Properties, Electronic Configuration, Atomic Spectrum, Uses.

Phosphorus
Phosphorus is 15th element of Periodic table with atomic number 15, atomic weight 30.973761. Phosphorus, symbol ‘P’, has a Simple Triclinic structure and Colorless color. Phosphorus is a other nonmetal element. Trivial name of Phosphorus is pentels, pnictogens*. Know everything about Phosphorus Facts, Physical Properties, Chemical Properties, Electronic configuration, Atomic and Crystal Structure.

History of Phosphorus
The element Phosphorus was discovered by Hennig Brand in year 1669 in Germany . Phosphorus derived its name from the Greek word phoosphoros, ‘carrying light’
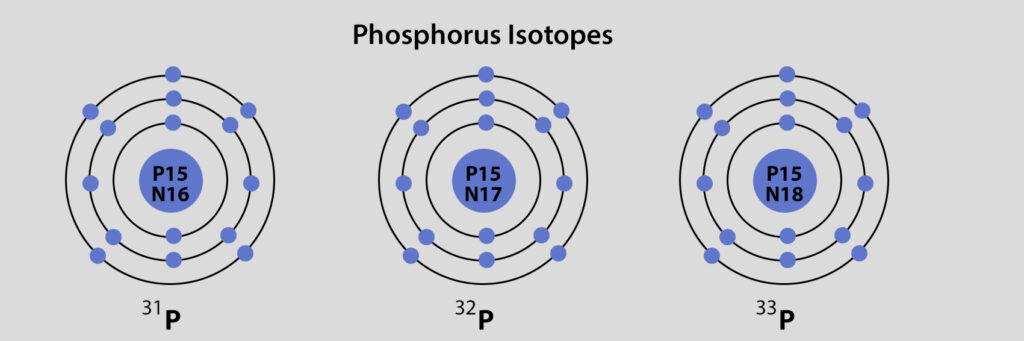
Phosphorus is a chemical element with symbol P and atomic number 15. As an element, phosphorus exists in two major forms—white phosphorus and red phosphorus—but due to its high reactivity, phosphorus is never found as a free element on Earth. Instead phosphorus-containing minerals are almost always present in their maximally oxidised state, as inorganic phosphate rocks.
How to Locate Phosphorus on Periodic Table
Periodic table is arranged by atomic number, number of protons in the nucleus which is same as number of electrons. The atomic number increases from left to right. Periodic table starts at top left ( Atomic number 1) and ends at bottom right (atomic number 118). Therefore you can directly look for atomic number 15 to find Phosphorus on periodic table.
Another way to read periodic table and locate an element is by using group number (column) and period number (row). To locate Phosphorus on periodic table look for cross section of group 15 and period 3 in the modern periodic table
Phosphorus Facts
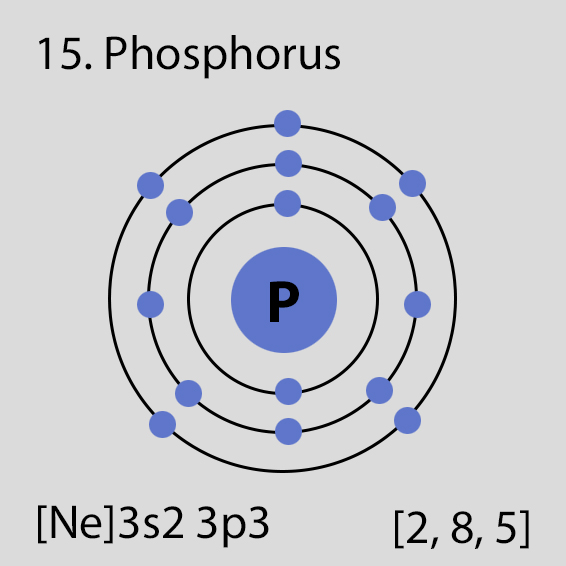
Phosphorus Atomic Structure and Orbital Properties
Phosphorus atoms have 15 electrons and the electronic shell structure is [2, 8, 5] with Atomic Term Symbol (Quantum Numbers) 4S3/2.
Element Properties
4S3/2
Atomic Structure of Phosphorus
Crystal Structure of Phosphorus
The solid state structure of Phosphorus is Simple Triclinic.
The Crystal structure can be described in terms of its unit Cell. The unit Cells repeats itself in three dimensional space to form the structure
The unit cell is represented in terms of its lattice parameters, which are the lengths of the cell edges Lattice Constants (a=1145, b=550.3 & c=1126.1 pm and the angles between them Lattice Angles (alpha=1.25384, beta=1.57725 and gamma=1.24896).
The symmetry properties of the crystal are described by the concept of space groups. All possible symmetric arrangements of particles in three-dimensional space are described by the 230 space groups (219 distinct types, or 230 if chiral copies are considered distinct.
Space Group Name
Space Group Number
Crystal Structure
P-1
2
Simple Triclinic

Ground State Electronic Configuration of Phosphorus - neutral Phosphorus atom
The ground state electronic configuration of Neutral Phosphorus atom is [Ne] 3s2 3p3. The portion of Phosphorus configuration that is equivalent to the noble gas of the preceding period, is abbreviated as [Ne]. For atoms with many electrons, this notation can become lengthy and so an abbreviated notation is used.This is important as it is the Valence electrons 3s2 3p3, electrons in the outermost shell that determine the chemical properties of the element.
Unabbreviated electronic configuration of neutral Phosphorus
Complete ground state electronic configuration for the Phosphorus atom, Unabbreviated electronic configuration
1s2 2s2 2p6 3s2 3p3
Regulatory and Health - Health and Safety Parameters and Guidelines
Phosphorus Chemical Properties : Phosphorus Ionization Energies and electron affinity
Phosphorus Physical & Elastic Properties
Phosphorus Electrical Properties
Phosphorus Magnetic Properties
Phosphorus Thermal Properties
Use of Phosphorus
The main use of phosphorus, in the form of concentrated phosphoric acid, in fertilizers. Phosphorus compounds are also used in baking powder, pesticides, detergents, and plasticisers. The main function of phosphorus is in the formation of bones and teeth. It plays an important role in how the body uses carbohydrates and fats. It is also needed for the body to make protein for the growth, maintenance, and repair of cells and tissues.