Hydrogen [H] – Element Details, Facts, Properties, Trends, Uses, Comparison with other elements

Hydrogen
Element 1 of Periodic table is Hydrogen with atomic number 1, atomic weight 1.00794. Hydrogen, symbol H, has a Simple Hexagonal structure and Colorless color. Hydrogen is a other nonmetal element. Trivial name of Hydrogen is alkali metals*. Know everything about Hydrogen

Hydrogen History
The element Hydrogen was discovered by Henry Cavendish in year 1766 in United Kingdom . Hydrogen derived its name from the Greek elements hydro- and -gen meaning ‘water-forming’
Hydrogen is a chemical element with chemical symbol H and atomic number 1. With an atomic weight of 1.00794 u, hydrogen is the lightest element on the periodic table. Its monatomic form (H) is the most abundant chemical substance in the Universe, constituting roughly 75% of all baryonic mass.
How to Locate Hydrogen on Periodic Table
Periodic table is arranged by atomic number, number of protons in the nucleus which is same as number of electrons. The atomic number increases from left to right. Periodic table starts at top left ( Atomic number 1) and ends at bottom right (atomic number 118). Therefore you can directly look for atomic number 1 to find Hydrogen on periodic table.
Another way to read periodic table and locate an element is by using group number (column) and period number (row). To locate Hydrogen on periodic table look for cross section of group 1 and period 1 in the modern periodic table
Hydrogen Facts
Hydrogen Atomic and Orbital Properties
Hydrogen atoms have 1 electrons and the electronic shell structure is [1] with Atomic Term Symbol (Quantum Numbers) 2S1/2.

Properties
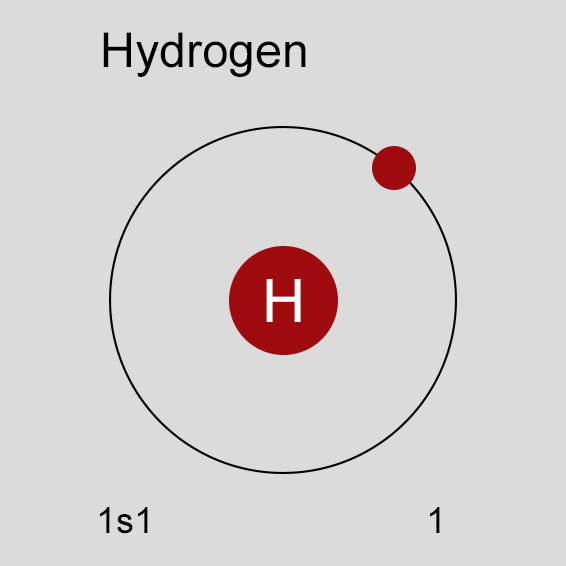
Atomic Structure of Hydrogen
Ground State Electronic Configuration of Hydrogen – neutral Hydrogen atom
The ground state electronic configuration of Neutral Hydrogen atom is 1s1. The portion of Hydrogen configuration that is equivalent to the noble gas of the preceding period, is abbreviated as 1s1]. For atoms with many electrons, this notation can become lengthy and so an abbreviated notation is used.This is important as it is the Valence electrons 1s1, electrons in the outermost shell that determine the chemical properties of the element.
Unabbreviated electronic configuration of neutral Hydrogen
Complete ground state electronic configuration for the Hydrogen atom, Unabbreviated electronic configuration
1s1
Atomic Spectrum of Hydrogen
