- History of Chlorine
- How to Locate Chlorine on Periodic Table
- Chlorine Facts
- Chlorine Atomic Structure and Orbital Properties
- Element Properties
- Atomic Structure of Chlorine
- Ground State Electronic Configuration of Chlorine - neutral Chlorine atom
- Atomic Spectrum of Chlorine
- Chlorine Chemical Properties : Chlorine Ionization Energies and electron affinity
- Use of Chlorine
Chlorine[Cl] – Element Details, History, Atomic Structure, Facts, Properties, Electronic Configuration, Atomic Spectrum, Uses of Chlorine.

Chlorine is the 17th Element of Periodic table with atomic number 17, atomic weight 35.453. Chlorine symbol Cl, has a Base Centered Orthorhombic structure and Yellow color. Chlorine is a Halogens element. It is part of group 17 (fluorine family). Know everything about Chlorine Facts, Properties, Electronic configuration, Atomic and Crystal Structure.

History of Chlorine
The element Chlorine was discovered by W. Scheele in year 1774 in Sweden. Chlorine was first isolated by W. Scheele in 1774. Chlorine derived its name from the Greek word chloros, meaning ‘greenish yellow’.
Obtained it from hydrochloric acid, but thought it was an oxide. Only in 1808 did Humphry Davy recognize it as an element.
How to Locate Chlorine on Periodic Table
Elements are arranged in periodic table by atomic number, number of protons in the nucleus which is same as number of electrons. Periodic table starts at top left ( Atomic number 1) and ends at bottom right (atomic number 118). Therefore you can directly look for atomic number 17 to find Chlorine on periodic table.
Another way to read periodic table and locate an element is by using group number (column) and period number (row). To locate Chlorine on periodic table look for cross section of group 17 and period 3 in the modern periodic table.
Chlorine Facts
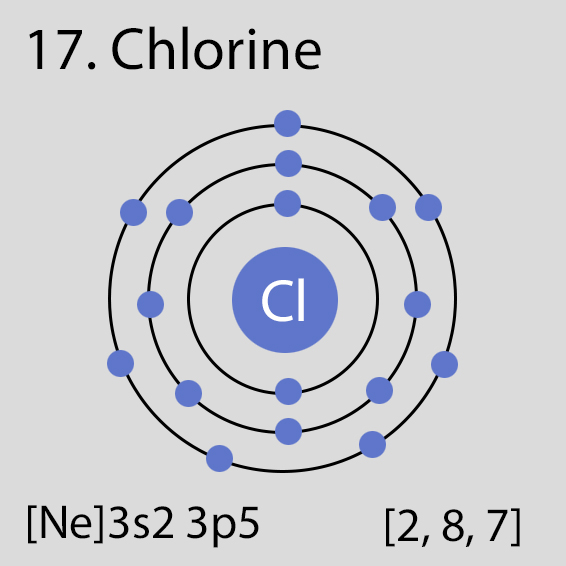
Chlorine Atomic Structure and Orbital Properties
Chlorine atoms have 17 electrons and the electronic shell structure is [2, 8, 7] with Atomic Term Symbol (Quantum Numbers) 2P3/2.
Element Properties
Atomic Structure of Chlorine
Ground State Electronic Configuration of Chlorine - neutral Chlorine atom
Abbreviated electronic configuration of Chlorine
The ground state abbreviated electronic configuration of Neutral Chlorine atom is [Ne] 3s2 3p5. The portion of Chlorine configuration that is equivalent to the noble gas of the preceding period, is abbreviated as [Ne]. For atoms with many electrons, this notation can become lengthy and so an abbreviated notation is used. This is important as it is the Valence electrons 3s2 3p5, electrons in the outermost shell that determine the chemical properties of the element.
Unabbreviated electronic configuration of neutral Chlorine
Complete ground state electronic configuration for the Chlorine atom, Unabbreviated electronic configuration
1s2 2s2 2p6 3s2 3p5
Electrons are filled in atomic orbitals as per the order determined by the Aufbau principle, Pauli Exclusion Principle and Hund’s Rule.
Atomic Spectrum of Chlorine

Chlorine Chemical Properties : Chlorine Ionization Energies and electron affinity
Use of Chlorine
Chlorine is one of the most useful element in the World. Mostly used in:
- Water. Chlorine chemistry helps keep drinking water and swimming pools safe.
- Household Disinfectant.
- Food.
- Healthcare.
- Energy and Environment.
- Advanced Technology.
- Building and Construction.
- Defense and Law Enforcement.